
Bracco offers you a broad range of options so you have choice and flexibility in the way you treat patients.
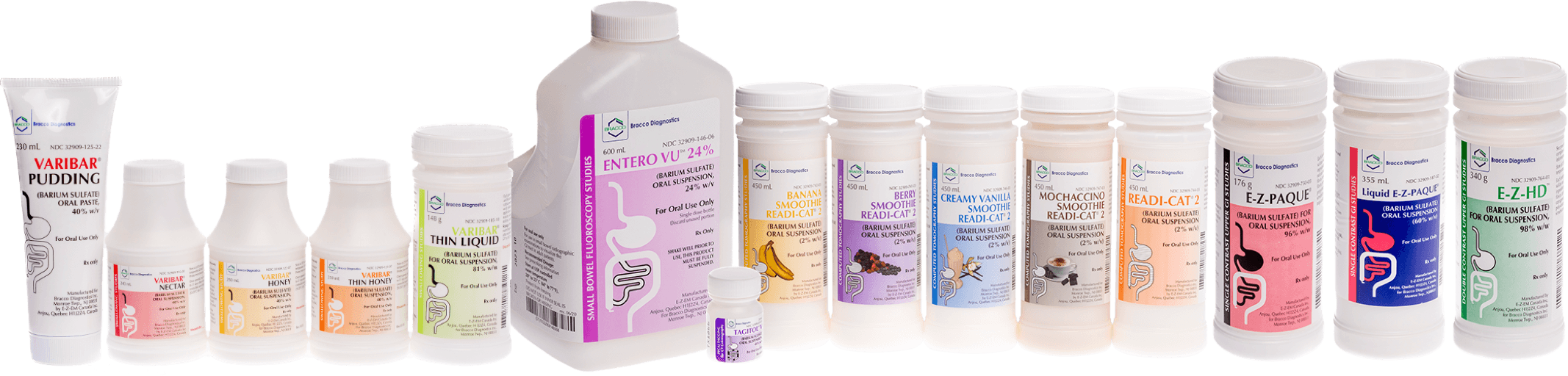
Every barium product above is a Bracco product – how many do you use?
The Bracco Legacy
- Bracco has been the leading provider of barium contrast agents since 2008
- Since then, we’ve made multiple NDA submissions and secured FDA approvals to ensure products remain available
- Our products are manufactured in North America using pharmaceutical grade, naturally sourced barium sulfate in an FDA–registered API facility

Product Availability
- Today, we offer a broad range of barium products for all of your GI imaging needs
- Our products are widely available and sold by over 35 national and regional distribution partners
Service & Support
- Dedicated Bracco representatives can help you choose the right products and answer your availability questions
- Bracco supports the barium community through CE programs, education grants, and professional societies

The individuals who appear are for illustrative purposes. All persons depicted are models and not real patients or healthcare professionals. Images are for illustrative purposes only.
Browse available Radiology CE courses
Click To Find a Bracco Barium Rep Near You
ENTERO VU™ 24% (barium sulfate) oral suspension, 24% w/v
Indication
ENTERO VU™ (barium sulfate) oral suspension is indicated for use in small bowel radiographic examinations to visualize the gastrointestinal (GI) tract in adult patients.
Contraindications
ENTERO VU is contraindicated in patients with known or suspected perforation of the GI tract, known obstruction of the GI tract, high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis, high risk of aspiration such as those with known or suspected tracheo-esophageal fistula or obtundation, or known severe hypersensitivity to barium sulfate or any of the excipients.
Warnings and Precautions
Hypersensitivity reactions
Barium sulfate preparations contain a number of excipients, including natural and artificial flavors and may induce serious hypersensitivity reactions. The manifestations include hypotension, bronchospasm and other respiratory impairments, and dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, food allergies, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction.
Intra-abdominal Barium Leakage
The use of ENTERO VU 24% is contraindicated in patients at high risk of perforation of the GI tract and may result in leakage of barium from the GI tract in the presence of conditions that increase the risk of perforation such as known carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, diverticulitis, and in patients with a severe stenosis at any level of the GI tract, especially if it is distal to the stomach. Barium leakage has been associated with peritonitis and granuloma formation.
Delayed Gastrointestinal Transit and Obstruction
Orally administered barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with development of baroliths (inspissated barium associated with feces) and may cause abdominal pain, appendicitis, bowel obstruction, or perforation. Patients with the following conditions are at higher risk for developing obstruction or baroliths: severe stenosis at any level of the GI tract, impaired GI motility, electrolyte imbalance, dehydration, on a low residue diet, on medications that delay GI motility, constipation, and the elderly. To reduce the risk of delayed GI transit and obstruction, patients should maintain adequate hydration after the barium sulfate procedure.
Aspiration Pneumonitis
The use of ENTERO VU 24% is contraindicated in patients with trachea-esophageal fistula. Oral administration of barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanism. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis. In patients at risk for aspiration, begin the procedure with a small ingested volume of ENTERO VU 24%. Monitor the patient closely for aspiration, discontinue administration of ENTERO VU 24% if aspiration is suspected, and monitor for development of aspiration pneumonitis.
Systemic Embolization
Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a “barium embolus” leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia and prolonged severe hypotension. Although this complication is uncommon after oral administration of a barium sulfate suspension, monitor patients for potential intravasation when administering barium sulfate.
Risk with Hereditary Fructose Intolerance
ENTERO VU 24% contains sorbitol which may cause severe symptoms if ingested by patients with hereditary fructose intolerance. Severe symptoms may include the following: vomiting, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia, and kidney failure. Before administration of ENTERO VU 24% assess patients for a history of hereditary fructose intolerance and avoid use in these patients.
The following adverse reactions have been identified from spontaneous reporting or clinical studies of barium sulfate administered orally and may not reliably estimate the frequency because the reactions are reported voluntarily from a population of uncertain size:
• Nausea, vomiting, diarrhea and abdominal cramping
• Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for Full Prescribing Information for ENTERO VU 24% (barium sulfate) oral suspension.
ENTERO VU 24% is manufactured for Bracco Diagnostics Inc., Princeton, NJ 08831 by E-Z-EM Canada Inc.
ENTERO VU is a trademark of E-Z-EM, Inc.
E-Z-DISK™ (Barium Sulfate Tablets) 700 mg
Indication
E-Z-DISK™ (Barium Sulfate Tablets) 700 mg is for use in radiography of the esophagus, for detection of esophageal strictures.
IMPORTANT SAFETY INFORMATION
Contraindications
- E-Z-DISK is contraindicated in patients with known gastric or intestinal perforation or hypersensitivity to barium sulfate formulations.
Warnings and Precautions
Hypersensitivity Reactions
Severe allergic reactions of an anaphylactic nature have been reported following administration of barium sulfate contrast agents. A history of bronchial asthma, atopy, as evidenced by hay fever and eczema, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction. Use with caution in patients with complete or nearly complete esophageal or gastric obstruction.
ADVERSE REACTIONS
Adverse reactions such as nausea, vomiting, diarrhea, and abdominal cramping accompanying the use of barium sulfate suspensions are infrequent, usually mild, and generally do not occur with this product. Procedural complications are rare, but may include aspiration pneumonitis, granuloma formation, intravasation, embolization and peritonitis following intestinal perforation, vasovagal and syncopal episodes, and fatalities.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for E-Z-DISK™ (Barium Sulfate Tablets).
E-Z-DISK is manufactured for E-Z-EM, Inc., a subsidiary of Bracco Diagnostics Inc., Princeton, NJ 08831.
E-Z-DISK is a trademark of E-Z-EM, Inc.
E-Z-HD™ (BARIUM SULFATE) FOR ORAL SUSPENSION, 98% w/w
INDICATION:
E-Z-HD™ (BARIUM SULFATE) FOR ORAL SUSPENSION is indicated in double-contrast radiographic examinations of the esophagus, stomach, and duodenum, to help visualize the gastrointestinal tract (GI) in patients 12 years and older.
IMPORTANT SAFETY INFORMATION
Contraindications
- E-Z-HD is contraindicated in patients with:
- known or suspected perforation of the GI tract
- known obstruction of the GI tract
- at high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post-GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis
- high risk of aspiration such as those with prior aspiration, pediatric patients with tracheoesophageal fistula, or obtundation
- known severe hypersensitivity to barium sulfate or any of the excipients of E-Z-HD
Warnings and Precautions
Hypersensitivity Reactions
Barium sulfate preparations contain a number of excipients, including natural and artificial flavors, and may induce serious hypersensitivity reactions which include hypotension, bronchospasm and other respiratory impairments, and dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction.
Intra-abdominal Barium Leakage
E-Z-HD is contraindicated in patients at high risk of perforation to the GI tract. Administration of E-Z-HD may result in leakage of barium from the GI tract in the presence of conditions such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, diverticulitis, and in patients with severe stenosis at any level of the GI tract, especially distal to the stomach. Barium leakage has been associated with peritonitis and granuloma formation.
Delayed Gastrointestinal Transit and Obstruction
Oral barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with development of baroliths (inspissated barium associated with feces) and may lead to abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with severe stenosis at any level of the GI tract, impaired GI motility, electrolyte imbalance, dehydration, on a low residue diet, on medications that delay GI motility, constipation, pediatric patients with cystic fibrosis, or Hirschsprung disease, and the elderly are at higher risk for developing obstruction or baroliths. Patients should maintain adequate hydration during and in the days following a barium sulfate procedure. Consider the administration of laxatives.
Aspiration Pneumonitis
E-Z-HD is contraindicated in patients at high risk of aspiration. Oral barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanisms. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis. In patients at risk for aspiration, begin the procedure with a small ingested volume of E-Z-HD. Discontinue administration of E-Z-HD immediately if aspiration is suspected.
Systemic Embolization
Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a “barium embolus” leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia, and prolonged severe hypotension. This complication is exceedingly uncommon after oral administration, monitor patients for potential intravasation when administering barium sulfate.
Risk with Hereditary Fructose Intolerance
E-Z-HD contains sorbitol which may cause severe symptoms in patients with hereditary fructose intolerance including severe symptoms of vomiting, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia, and kidney failure. Before administration of E-Z-HD assess patients for a history of hereditary fructose intolerance and avoid use in these patients.
ADVERSE REACTIONS
The following adverse reactions have been identified from spontaneous reporting or clinical studies of orally administered barium sulfate:
Nausea, vomiting, diarrhea, and abdominal cramping
Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for E-Z-HD™ (BARIUM SULFATE FOR ORAL SUSPENSION).
E-Z-HD is manufactured by E-Z-EM Canada Inc., for E-Z-EM, Inc., a subsidiary of Bracco Diagnostics Inc., Princeton, NJ 08831.
E-Z-HD is a trademark of E-Z-EM, Inc.
E-Z-PAQUE® (barium sulfate) for oral suspension
INDICATION:
E-Z-PAQUE (barium sulfate) for oral suspension is indicated in adults and pediatrics for use in single contrast radiographic examinations of the esophagus, stomach, duodenum, and small bowel to visualize the gastrointestinal tract (GI).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
- E-Z-PAQUE is contraindicated in patients with:
- known or suspected perforation of the GI tract
- known obstruction of the GI tract
- high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post-GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis
- high risk of aspiration such as those with prior aspiration, tracheoesophageal fistula, or obtundation
- known severe hypersensitivity to barium sulfate or any of the excipients of E-Z-PAQUE
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Barium sulfate preparations contain excipients, including natural and artificial flavors, and may induce serious hypersensitivity reactions which include hypotension, bronchospasm and other respiratory impairments, and dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions.
Intra-abdominal Barium Leakage
Administration of E-Z-PAQUE may result in leakage of barium from the GI tract in the presence of conditions such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, diverticulitis, and in patients with severe stenosis at any level of the GI tract, especially distal to the stomach. Barium leakage has been associated with peritonitis and granuloma formation.
Delayed Gastrointestinal Transit and Obstruction
Oral barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with the development of baroliths (inspissated barium associated with feces) and may cause abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with severe stenosis at any level of the GI tract, impaired GI motility, electrolyte imbalance, dehydration, on a low residue diet, on medications that delay GI motility, constipation, cystic fibrosis, Hirschsprung disease, and the elderly are at higher risk for developing obstruction or baroliths. Maintain adequate hydration during and in the days following a barium sulfate procedure. Consider the administration of laxatives.
Aspiration Pneumonitis
Oral barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanisms. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis. In patients at risk for aspiration, begin the procedure with a small, ingested volume of E-Z-PAQUE. Discontinue administration of E-Z-PAQUE immediately if aspiration is suspected.
Systemic Embolization
Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a “barium embolus” leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia, and prolonged severe hypotension. This complication is exceedingly uncommon after oral administration, monitor patients for potential intravasation when administering barium sulfate.
Risk with Hereditary Fructose Intolerance
E-Z-PAQUE contains sorbitol which may cause severe symptoms in patients with hereditary fructose intolerance including severe symptoms of vomiting, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia, and kidney failure. Before administration of E-Z-PAQUE assess patients for a history of hereditary fructose intolerance and avoid use in these patients.
ADVERSE REACTIONS
The following adverse reactions have been identified from spontaneous reporting or clinical studies of orally administered barium sulfate:
- Nausea, vomiting, diarrhea, and abdominal cramping
- Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for E-Z-PAQUE® (barium sulfate) for oral suspension.
E-Z-PAQUE is manufactured by E-Z-EM Canada Inc., for E-Z-EM, Inc., a subsidiary of Bracco Diagnostics Inc., Princeton, NJ 08831.
E-Z-PAQUE is a registered trademark of E-Z-EM, Inc.
Liquid E-Z-PAQUE® (BARIUM SULFATE) ORAL SUSPENSION (60% w/v)
INDICATION:
Liquid E-Z-PAQUE (barium sulfate) oral suspension is indicated in adults and pediatrics for use in single contrast radiographic examinations of the esophagus, stomach, and small bowel to visualize the gastrointestinal tract (GI).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Liquid E-Z-PAQUE is contraindicated in patients with:
- known or suspected perforation of the GI tract
- known obstruction of the GI tract
- high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post-GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis
- high risk of aspiration such as those with prior aspiration, tracheoesophageal fistula, or obtundation
- known severe hypersensitivity to barium sulfate or any of the excipients of Liquid E-Z-PAQUE.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Barium sulfate preparations contain excipients, including natural and artificial flavors, and may induce serious hypersensitivity reactions which include hypotension, bronchospasm and other respiratory impairments, and dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions.
Intra-abdominal Barium Leakage
Administration of Liquid E-Z-PAQUE may result in leakage of barium from the GI tract in the presence of conditions such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, diverticulitis, and in patients with severe stenosis at any level of the GI tract, especially distal to the stomach. Barium leakage has been associated with peritonitis and granuloma formation.
Delayed Gastrointestinal Transit and Obstruction
Oral barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with the development of baroliths (inspissated barium associated with feces) and may cause abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with severe stenosis at any level of the GI tract, impaired GI motility, electrolyte imbalance, dehydration, on a low residue diet, on medications that delay GI motility, constipation, cystic fibrosis, Hirschsprung disease, and the elderly are at higher risk for developing obstruction or baroliths. Maintain adequate hydration during and in the days following a barium sulfate procedure.
Aspiration Pneumonitis
Oral barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanisms. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis. In patients at risk for aspiration, begin the procedure with a small, ingested volume of Liquid E-Z-PAQUE. Discontinue administration of Liquid E-Z-PAQUE immediately if aspiration is suspected.
Systemic Embolization
Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a “barium embolus” leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia, and prolonged severe hypotension. Although this complication is exceedingly uncommon after oral administration, monitor patients for potential intravasation when administering barium sulfate.
Risk with Hereditary Fructose Intolerance
Liquid E-Z-PAQUE contains sorbitol which may cause symptoms in patients with hereditary fructose intolerance including severe symptoms of vomiting, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia, and kidney failure. Before administration of Liquid E-Z-PAQUE assess patients for a history of hereditary fructose intolerance and avoid use in these patients.
ADVERSE REACTIONS
The following adverse reactions have been identified from spontaneous reporting or clinical studies of orally administered barium sulfate:
- Nausea, vomiting, diarrhea, and abdominal cramping
- Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, and vasovagal and syncopal episodes.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for Full Prescribing Information for Liquid E-Z-PAQUE® (barium sulfate) oral suspension (60% w/v).
Liquid E-Z-PAQUE is manufactured by E-Z-EM Canada Inc., for E-Z-EM, Inc., a subsidiary of Bracco Diagnostics Inc., Princeton, NJ 08831.
E-Z-PAQUE is a registered trademark of E-Z-EM, Inc.
READI-CAT® 2 (barium sulfate) oral suspension
READI-CAT® 2 SMOOTHIE (barium sulfate) oral suspension
INDICATION
READI-CAT® 2 and READI-CAT® 2 SMOOTHIE (barium sulfate) oral suspension are indicated for use in computed tomography (CT) of the abdomen to delineate the gastrointestinal (GI) tract in adult and pediatric patients.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
READI-CAT 2 products are contraindicated in patients:
- with known or suspected perforation of the GI tract
- with known obstruction of the GI tract
- at high risk of GI perforation such as those with a recent prior GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to pelvis
- at high risk of aspiration such as those with prior aspiration, tracheo-esophageal fistula, or obtundation
- known severe hypersensitivity to barium sulfate or any of the excipients of READI-CAT 2 or READI-CAT 2 SMOOTHIES
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Barium sulfate preparations contain a number of excipients, including natural and artificial flavors, and may induce serious hypersensitivity reactions including hypotension, bronchospasm, and other respiratory impairments, dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction.
Intra-abdominal Barium Leakage The use of READI-CAT 2 products is contraindicated in patients at high risk of perforation of the GI tract. Administration of READI-CAT 2 products may result in leakage of barium from the GI tract in the presence of conditions such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, or diverticulitis, and in patients with a severe stenosis at any level of the GI tract, especially if it is distal to the stomach. The barium leakage has been associated with peritonitis and granuloma formation.
Delayed Gastrointestinal Transit and Obstruction Orally administered barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with development of baroliths (inspissated barium associated with feces) and may lead to abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with severe stenosis at any level of the GI tract, impaired GI motility, electrolyte imbalance, dehydration, on a low residue diet, taking medications that delay GI motility, and constipation, pediatric patients with cystic fibrosis or Hirschsprung disease, and the elderly are at higher risk for obstruction or baroliths. To reduce the risk of delayed GI transit and obstruction, patients should maintain adequate hydration following a barium sulfate procedure.
Aspiration Pneumonitis The use of READI-CAT 2 products is contraindicated in patients at high risk of aspiration. Oral administration of barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanism. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis. In patients at risk for aspiration, begin the procedure with a small, ingested volume of READI-CAT 2 products. Discontinue administration of READI-CAT 2 products immediately if aspiration is suspected.
Systemic Embolization Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a “barium embolus” leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia, and prolonged severe hypotension. Although this complication is exceedingly uncommon after oral administration of barium sulfate suspension, monitor patients for potential intravasation when administering barium sulfate.
Risk with Hereditary Fructose Intolerance READI-CAT 2 contains sorbitol which may cause severe reactions if ingested by patients with hereditary fructose intolerance, such as vomiting, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia, and kidney failure. Before administration of READI-CAT 2 assess patients for a history of hereditary fructose intolerance and avoid use in these patients.
ADVERSE REACTIONS
Most Common Adverse Events include nausea, vomiting, diarrhea, and abdominal cramping Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for READI-CAT® 2 products.
READI-CAT 2 is manufactured for Bracco Diagnostics Inc., Princeton, NJ 08831 by E-Z-EM Canada Inc.
READI-CAT is a registered trademark of E-Z-EM, Inc.
TAGITOL™ V (barium sulfate) oral suspension
INDICATION
TAGITOL™ V (barium sulfate) oral suspension is indicated for use in adult patients for use in computed tomography (CT) colonography as a fecal tagging agent.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
TAGITOL V is contraindicated in patients with:
- known or suspected perforation of the gastrointestinal (GI) tract
- known obstruction of the GI tract
- high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis
- high risk of aspiration such as those with prior aspiration, tracheo-esophageal fistula, or obtundation
- known hypersensitivity to barium sulfate or any of the excipients of TAGITOL V.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Barium sulfate preparations contain a number of excipients, including natural and artificial flavors, and may induce serious hypersensitivity reactions. The manifestations include hypotension, bronchospasm, and other respiratory impairments, dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction.
Intra-abdominal Barium Leakage The use of TAGITOL V is contraindicated in patients at high risk of perforation of the GI tract and may result in leakage of barium from the GI tract in the presence of conditions that increase the risk of perforation such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, or diverticulitis, and in patients with a severe stenosis at any level of the gastrointestinal tract, especially if it is distal to the stomach. The barium leakage has been associated with peritonitis and granuloma formation.
Delayed Gastrointestinal Transit and Obstruction Orally administered barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with development of baroliths (inspissated barium associated with feces) and may lead to abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with severe stenosis at any level of the GI tract, impaired gastrointestinal motility, electrolyte imbalance, dehydration, on a low residue diet, on medications that delay GI motility, constipation, cystic fibrosis, Hirschsprung disease, and the elderly are at higher risk for developing obstruction or barolith. Patients should maintain adequate hydration to reduce the risk of delayed GI transit and obstruction.
Aspiration Pneumonitis The use of TAGITOL V is contraindicated in patients at high risk of aspiration. Oral administration of barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanism. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis.
Systemic Embolization Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a “barium embolus” leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia, and prolonged severe hypotension. Although this complication is exceedingly uncommon after oral administration, monitor patients for potential intravasation.
ADVERSE REACTIONS
Adverse reactions include Nausea, vomiting, diarrhea, and abdominal cramping. Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for TAGITOL™ V (barium sulfate) oral suspension.
TAGITOL V is manufactured by E-Z-EM Canada Inc., for E-Z-EM, Inc., a subsidiary of Bracco Diagnostics Inc., Princeton, NJ 08831.
TAGITOL is a trademark of E-Z-EM, Inc.
VARIBAR® (barium sulfate)
INDICATIONS
VARIBAR® THIN HONEY (barium sulfate) oral suspension, VARIBAR® NECTAR (barium sulfate) oral suspension, and VARIBAR® THIN LIQUID (barium sulfate) oral suspension are radiographic contrast agents indicated for use in modified barium swallow examinations to evaluate the oral and pharyngeal function and morphology in adult and pediatric patients.
VARIBAR® HONEY (barium sulfate) oral suspension and VARIBAR® PUDDING (barium sulfate) oral paste are radiographic contrast agents indicated for use in modified barium swallow examinations to evaluate the oral and pharyngeal function and morphology in adult and pediatric patients 6 months of age and older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
These products should not be used in patients with known or suspected perforation of the gastrointestinal (GI) tract; known obstruction of the GI tract; high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis; high risk of aspiration such as those with known or suspected tracheo-esophageal fistula or obtundation; known severe hypersensitivity to barium sulfate or any of the excipients of the product used.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Barium sulfate preparations contain a number of excipients, including natural and artificial flavors, and may induce serious hypersensitivity reactions. The manifestations include hypotension, bronchospasm and other respiratory impairments, and dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, food allergies, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction.
Intra-abdominal Barium Leakage
The use of VARIBAR PRODUCTS is contraindicated in patients at high risk of perforation of the GI tract. Administration of VARIBAR PRODUCTS may result in leakage of barium from the GI tract in the presence of conditions such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, or diverticulitis, and in patients with a severe stenosis at any level of the GI tract, especially if it is distal to the stomach. The barium leakage has been associated with peritonitis and granuloma formation.
Delayed Gastrointestinal Transit and Obstruction
Orally administered barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with development of baroliths (inspissated barium associated with feces) and may lead to abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with the following conditions are at higher risk for developing obstruction or baroliths: severe stenosis at any level of the GI tract, impaired GI motility, electrolyte imbalance, dehydration, on a low residue diet, taking medications that delay GI motility, constipation, pediatric patients with cystic fibrosis or Hirschsprung disease, and the elderly. To reduce the risk of delayed GI transit and obstruction, patients should maintain adequate hydration after the barium sulfate procedure. When administering VARIBAR PUDDING, consider the administration of laxatives.
Aspiration Pneumonitis
The use of VARIBAR PRODUCTS is contraindicated in patients with trachea-esophageal fistula. Oral administration of barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanism. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis. In patients at risk for aspiration, begin the procedure with a small ingested volume of VARIBAR PRODUCTS. Monitor the patient closely for aspiration, discontinue administration of VARIBAR PRODUCTS if aspiration is suspected, and monitor for development of aspiration pneumonitis.
Systemic Embolization
Barium sulfate products may occasionally intravasate into the venous drainage of the GI tract and enter the circulation as a “barium embolus” leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia and prolonged severe hypotension. Although this complication is exceedingly uncommon after oral administration of a barium sulfate suspension, monitor patients for potential intravasation when administering barium sulfate.
ADVERSE REACTIONS
The most common adverse reactions are nausea, vomiting, diarrhea, and abdominal cramping. Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for VARIBAR® THIN LIQUID (barium sulfate) oral suspension.
Please click here for full Prescribing Information for VARIBAR® THIN HONEY (barium sulfate) oral suspension.
Please click here for full Prescribing Information for VARIBAR® NECTAR (barium sulfate) oral suspension.
Please click here for full Prescribing Information for VARIBAR® HONEY (barium sulfate) oral suspension.
Please click here for full Prescribing Information for VARIBAR® PUDDING (barium sulfate) oral paste.
VARIBAR is manufactured by E-Z-EM Canada Inc., for E-Z-EM, Inc., a subsidiary of Bracco Diagnostics Inc., Princeton, NJ 08831.
VARIBAR is a registered trademark of E-Z-EM, Inc.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
ENTERO VU™ (barium sulfate) oral suspension, 24% w/v
Indication
ENTERO VU™ (barium sulfate) oral suspension is indicated for use in small bowel radiographic examinations to visualize the gastrointestinal (GI) tract in adult patients.
Contraindications
ENTERO VU is contraindicated in patients with known or suspected perforation of the GI tract, known obstruction of the GI tract, high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis, high risk of aspiration such as those with known or suspected tracheo-esophageal fistula or obtundation, or known severe hypersensitivity to barium sulfate or any of the excipients.
![]()